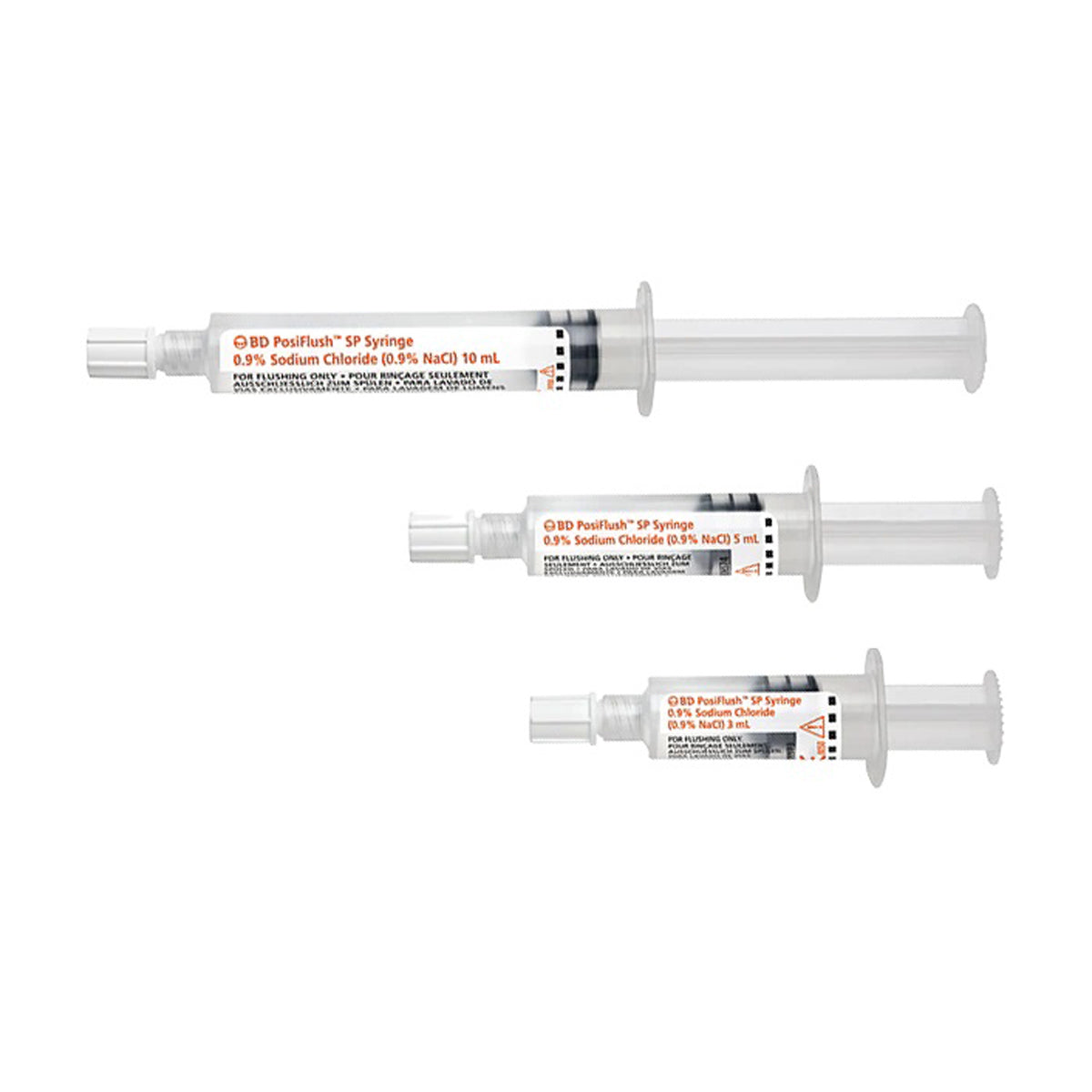
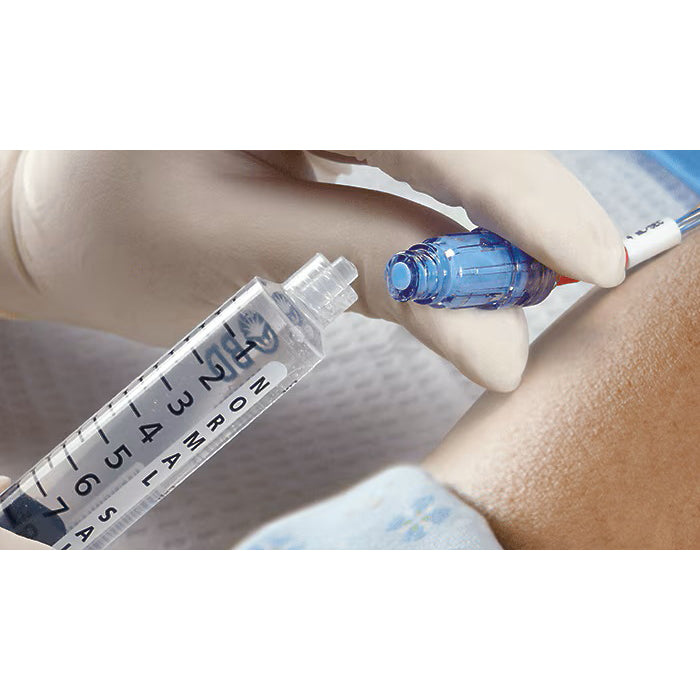
BD PosiFlush SP prefilled saline syringes are single use saline flush syringes designed for the routine flushing of indwelling vascular access devices outside the sterile field. Each syringe is prefilled with 0.9% sodium chloride and features a sterile fluid path, supporting catheter patency, medication delivery and infection prevention as part of standard IV care.
The PosiFlush SP range uses a standard 10 mL syringe barrel across the 3 mL, 5 mL and 10 mL syringes. This helps limit injection pressure compared with smaller diameter syringes and supports compliance with PICC and central line flushing recommendations while maintaining a familiar feel for clinicians.
Key features and benefits
- Ready to use saline flush – prefilled with 0.9% sodium chloride, reducing on-ward preparation, variability and the risk of contamination linked to manually drawn flush syringes.
- Sterile fluid path design – SP sterile fluid pathway with BD Luer-Lok tip and secure tip cap helps maintain sterility of the fluid path and supports closed system practice when flushing vascular access devices.
- Standard 10 mL barrel diameter – all saline volumes use a 10 mL diameter syringe to limit flushing pressures and help reduce the risk of catheter or vessel damage compared with standard small diameter syringes.
- Clear, safety focused labelling – bold printed labelling for volume, solution, lot number and expiry date supports fast identification, helps distinguish saline from medications and reduces the risk of flush related medication errors.
- Latex free and preservative free – saline solution is preservative free and the device is latex free, with no BPA or DEHP in the material formulation, making it suitable for use with most patient groups including those with common sensitivities.
- Time and waste reduction – removes the need for ampoules, vials and drawing up needles, which supports more efficient workflows, reduces sharps handling and can help lower consumable waste and disposal costs.
- Assured quality and compliance – steam sterilised, Class III medical device with CE 0050 marking and a three year shelf life, supporting reliable performance and straightforward stock rotation across busy clinical areas.